Defining Humane Endpoints
BACKGROUND
Animals used in biomedical research may experience pain or distress as part of the experimental protocol. Researchers have a moral, ethical, and legal obligation to minimize pain and distress in pursuit of reliable, reproducible data. This is best achieved by the use of humane endpoints, or criteria used to define when an animal is to be removed from a study. Appropriate humane endpoints provide opportunities for refinement and reduce confounding systemic effects which may increase the precision of the data.11 All studies must describe humane endpoints in their animal use protocol.
Definitions
- Experimental Endpoints - Endpoint of a study which occurs when the scientific aims and objectives have been reached (Guide p. 27).
- Humane Endpoints - Predetermined physiological or behavioral signs that define the point at which an experimental animal's pain and/or distress is reduced or terminated through actions such as halting a procedure, providing appropriate treatment or analgesia, or humane euthanasia.
- Morbidity - A condition of being unhealthy or diseased.
- Moribund - A severely debilitated clinical state that precedes imminent death.
IACUC Guidelines
Humane endpoints must be clearly defined and based on objective criteria (See table below for examples). They are study-specific and should be formulated through discussion with the PI, IACUC, and veterinarian while considering the following:
- Animal model and normal species-specific behaviors
- Scientific goals and experimental endpoints
- Expected and/or potential adverse effects (pain, distress, illness, death, etc.)
- Probable progression and timeline of adverse effects
- Earliest most predicative indicators of present or impending adverse effects
Monitoring for humane endpoints must be described in the protocol, including specific assessment criteria and the frequency of animal observation and assessment.
The action(s) to be taken when an animal reaches a humane endpoint must be described in the protocol and include either:
- Institution of appropriate treatment and either pausing study participation or permanent removal from study; or
- Humane euthanasia
Pilot studies may be necessary to determine humane endpoints, particularly when the effects of a treatment are unknown. A pilot study may help determine the morbidity, time course of effects, and frequency of monitoring necessary to establish endpoints.
The IACUC considers the following clinical signs as humane endpoints for animals exhibiting study adverse effects and/or spontaneous disease.
For an animal to continue on study while exhibiting these clinical signs, it must be described and approved in the IACUC protocol with alternative humane endpoints and appropriate monitoring plans; or undergoing an appropriate treatment prescribed by a DLAR veterinarian.
Failure to respond to treatment is considered an endpoint and the animal must be humanely euthanized.
| Body System | Clinical Signs |
|---|---|
| General/Systemic | Weight loss >20% of body weight or age-matched controls and/or a body condition score (BCS) <2/5 (Figure 1) Lateral recumbency, loss of righting reflex Moribundity Pain which fails to respond to analgesia Any condition that interferes with ability to eat/drink/ambulate Dehydration that fails to improve with fluid therapy within 48 hours Abdominal distension or ascites Anasarca (systemic edema) Excessive or prolonged hypothermia or hyperthermia Anemia (pallor), jaundice Tumor which exceeds 10% of the body weight; ulcerated, necrotic, or infected tumors Tumors that interfere with eating/drinking/ambulation Skeletal fractures Tail and/or appendage necrosis |
| Integumentary System | Dermatitis that fails to resolve with treatment Skin lesions with underlying muscle exposed Infected surgical incisions that fail to resolve with treatment |
| Ocular | Infections that fail to resolve with treatment Buphthalmia (enlargement of the eye) Exophthalmos (protrusion of the eye) |
| Respiratory system | Cyanosis Increased respiratory effort Abnormal respiratory rate Open-mouth breathing |
| Digestive system | Anorexia (lack or loss of appetite) or failure to drink Malocclusion Hematochezia/melena (blood in feces) |
| Reproductive system | Paraphimosis that fails to resolve with treatment Vaginal/uterine prolapse |
| Renal system | Anuria (lack of urine production) or inability to urinate due to trauma or obstruction |
| Nervous system | Seizures Ataxia/paresis/paralysis Head tilt/circling/rolling |
*Treatment of any of the above conditions must be directed by a DLAR veterinarian.
Death or Moribundity as an Experimental Endpoint
Every attempt should be made to recognize and humanely euthanize an animal prior to moribundity or death. However, there are some studies that require moribundity or mortality as an endpoint.
Signs of a moribund animal include, but are not limited to:
- Lateral recumbency or immobility
- Lack of response to stimulation
- Inability to eat or drink
- Hypothermia13
Studies that require death or moribundity as an endpoint must be classified as a pain and distress category E.
The protocol must include the following information:
- A robust scientific justification including:
- Specific alternatives or refinements that were considered.
- Why earlier humane endpoints cannot be employed?
- Whether animals will be humanely euthanized if moribund, and if not, what additional information can be gained from the interval between moribundity and death.
- Detailed monitoring plans
- Frequency of monitoring should increase with morbidity
Tools for humane endpoint evaluation:
- Body condition scoring systems
- Mouse body condition score
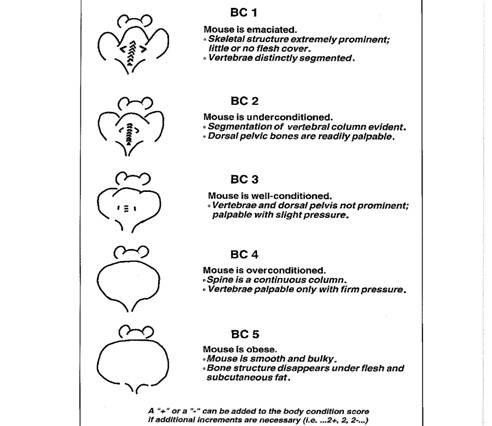
2. Pain scoring systems
a) Grimace scales
i) Mouse, Rat & Rabbit Grimace scales available

3. Humane endpoints scoring systems
Scoring templates should be adapted to individual studies and based on the scientific needs and expected outcomes in the animal model.
-
- Example Rubric for Humane Endpoints as adapted for a murine sepsis model8
| Variable | Score and Description |
|---|---|
| Appearance | 0 - Coat is smooth 1 - Patches of hair piloerected 2 - Majority of back is piloerected 3 - Piloerection may or may not be present, mouse appears "puffy" 4 - Piloerection may or may not be present, mouse appears emaciated |
| Level of consciousness | 0 - Mouse is active 1 - Mouse is active but avoids standing upright 2 - Mouse activity is noticeably slowed. The mouse is still ambulant. 3 - Activity is impaired. Mouse only moves when provoked, movements have a tremor 4 - Activity severely impaired. Mouse remains stationary when provoked, with possible tremor |
| Activity | 0 - Normal amount of activity. Mouse is any of: eating, drinking, climbing, running, fighting 1 - Slightly suppressed activity. Mouse is moving around bottom of cage 2 - Suppressed activity. Mouse is stationary with occasional investigative movements 3 - No activity. Mouse is stationary 4 - No activity. Mouse experiencing tremors, particularly in the hind legs |
| Response to stimulus | 0 - Mouse responds immediately to auditory stimulus or touch 1 - Slow or no response to auditory stimulus; strong response to touch (moves to escape) 2 - No response to auditory stimulus; moderate response to touch (moves a few steps) 3 - No response to auditory stimulus; mild response to touch (no locomotion) 4 - No response to auditory stimulus. Little to no response to touch. Cannot right itself if pushed over |
| Eyes | 0 - Open 1 - Eyes not fully open, possibly with secretions 2 - Eyes at least half closed, possibly with secretions 3 - Eyes half closed or more, possibly with secretions 4 - Eyes closed or milky |
| Respiration rate | 0 - Normal, rapid mouse respiration 1 - Slightly decreased respiration (rate not quantifiable by eye) 2 - Moderately reduced respiration (rate at the upper range of quantifying by eye) 3 - Severely reduced respiration (rate easily countable by eye, 0.5s between breaths) 4 - Extremely reduced respiration (>1s between breaths) |
| Respiration quality | 0 - Normal 1 - Brief periods of labored breathing 2 - Labored, no gasping' 3 - Labored with intermittent gasps 4 - Gasping |
Mice were euthanized at a score of 21 or if respiration rate or quality were 3
References
-
- Morton DB and Griffiths PHM (1985), Guidelines on the recognition of pain, distress, and discomfort in experimental animals and an hypothesis for assessment. Veterinary Record 116:431-43
- Canadian Council on Animal Care (1998), Humane Endpoints in Animal Experiments for Biomedical Research, teaching, and testing. Ottowa, Canada.
- Ullman-Cullere MH and Foltz CJ (1999) Body condition scoring: a rapid and accurate method for assessing health status of mice. Lab Anim SC 49:319-323.
- Kort WJ, Hekking-Weijma JM, TenKate MT, Sorm V, R. V. A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Lab Animal. 1998;32:260 269.
- Love JA, Booth CD, Boyd J. Remote temperature sensing for determining humane endpoints. In: 2nd World Congress on Alternatives and Animal Use in the Life Sciences. Utrecht, The Netherlands; 1996
- United Kingdom Coordinating Committee on Cancer Research. Guidelines for the welfare of animals in experimental neoplasia. London, UK; 1997.
- Wallace J. Humane Endpoints and Cancer Research. ILAR Journal 2000; 41(2).
- Shrum et al. BMC Research Notes 2014, 7:233
- IACUC Guideline Humane Endpoints for Laboratory Animals, University of Pennsylvania
- Guidelines for Endpoints in Animal Study Proposals. NIH OACU 27 April 2022. https://oacu.oir.nih.gov/system/files/media/file/2022-04/b13_endpoints_guidelines.pdf
- Workman P, Aboagye E, Balkwill F et al. (2010) Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102, 15551577. https://doi.org/10.1038/sj.bjc.6605642.
- National Centre for the Replacement Refinement & Reduction of Animals in Research (NC3Rs) Humane Endpoints 02 October 2015. < https://nc3rs.org.uk/3rs-resources/humane-endpoints>.
- Toth LA. (2000) Defining the Moribund Condition as an Experimental Endpoint for Animal Research. ILAR J. 41:72-79. https://academic.oup.com/ilarjournal/article/41/2/72/747769.
- Toth LA. (2018) Identifying and Implementing Endpoints for Geriatric Mice. Comp. Med. 68: 439-451.
Approved: October 2008
Revisions Approved: December 2012, May 2018, September 2022
Reviewed: October 2017