Food/Water Restriction, Regulation or Diet Manipulation
BACKGROUND
This guideline describes the recommendations for protocols that include the regulation or restriction of an animal's food and/or water. The Guide for the Care and Use of Laboratory Animals (the Guide, NRC 2011) states: "The objective when these studies are being planned and executed should be to use the least restriction necessary to achieve the scientific objective while maintaining animal well-being" (p. 31). The restriction or regulation of food/water must be described in detail in the protocol application and approved by the IACUC.
Definitions
- Regulation of food/water is defined as the scheduled access to food and/or water sources so an animal consumes as much as desired at regular intervals. For example, rats may have regulated access to food where the food is withheld for several hours in order for them to work for food as a reward in a behavioral test.
- Restriction of food/water is where the quantity or total volume consumed is strictly monitored and controlled (NRC 2003).
- Fasting is the removal of food (but not water) for a certain period of time prior to an experimental manipulation such as surgery, glucose tolerance testing, etc.
- Special Diet/Water is any experimental or non-standard diet or water type for the species.
- Treats/Food Rewards are a highly palatable food and/or fluid.
IACUC Policy
Protocols should propose minimal restriction or regulation necessary to achieve the scientific objective while maintaining animal well-being. Protocol applications must address the necessary level of regulation/restriction, the methods for assessing the health and well-being of the animals, the potential adverse consequences of regulation/restriction, and the steps to be taken to address the adverse health effects.
Animals must always have access to water unless justified and approved in the IACUC protocol.
Food regulation, restriction, or the use of a special diet requires assessment of minimum caloric requirements for the animal to maintain health. In the case of these conditioned-response research protocols, the use of a highly preferred food or fluid as positive reinforcement, instead of restriction/regulation, is recommended. If acclimation period for food regulation/restriction is needed this should be described in the IACUC protocol. Animals experiencing adverse effects due to food/water regulation/restriction must be evaluated by a veterinarian.
Special considerations
- Life stage (e.g., young, growing, pregnant, and lactating) and health status may affect the caloric requirement for maintaining animals. When determining restriction or regulation protocols these situations should be considered and appropriate monitoring implemented (e.g., weight assessment, comparison with expected growth curve).
Experimental/Special Diet and/or Water or Treats/Rewards
- Diets should be acquired from reputable vendors and must be kept, after opening, in labeled, clean, easily sanitized, vermin-proof containers
- Diets must be irradiated at the vendor.
- This is consistent with DLAR practices to reduce the potential for adventitious disease.
- If unable to be irradiated, DLAR veterinarians must be notified prior to use to discuss options.
- Diet must be used within six (6) months of the mill date, unless otherwise specified by the manufacturer.
- Special water bottles must be replaced weekly with fresh bottles and fresh water to prevent bacterial overgrowth and biofilm creation
- All treats and food rewards, not already approved as standard DLAR enrichment, must be listed and approved in the IACUC protocol.
Restriction/Fasting
- Pre-surgical fasting is the veterinary standard for larger species (cats, dogs, pigs) and does not need to be detailed in the IACUC protocol. Small research animals are not typically fasted before surgery. Therefore, pre-surgical fasting of rodents and rabbits must be justified in the IACUC protocol.
- Fasting may be necessary in preparation of a non-surgical experimental manipulation.
- The length of the fasting period must be specifically defined in the IACUC protocol.
- Experiments on rodents should be planned so that the fasting period starts in the morning, and the experimental manipulation occurs in the afternoon. This is because removing food "overnight" can have adverse effects as often rodents eat during the dark phase of the light cycle.
- If food does need to be removed prior to leaving the lab for the evening, a small portion of food that will be consumed over the next few hours (e.g., a few pellets) may be left on the cage floor.
- Any mammal without access to food for approximately more than 12 hours must be listed in Category E. Non-mammalian species will be addressed on a case-by-case basis. Additionally, if scientific needs require a weight loss of >20%, these animals must be listed in Category E.
- Animals without water for longer than 12 hours must be listed in Category E.
Recordkeeping
The following parameters should be measured to ensure that the nutritional needs of the animals are met. Animals must be assessed daily, and written records must be maintained for each animal to document these parameters. See Food and Water Restriction Log, as example.
- Mammalian animals must be weighed before experiments begin to establish a baseline weight. Weights must be recorded (at least) weekly during the periods of food/water regulation and time periods must be specified in the IACUC approved protocol. Non-mammalian animals will be assessed on a case-by-case basis.
- An adult mouse consumes an average of 12 g/100 g body weight of food per day, whereas an adult rat will consume an average of 5g/100g body weight. An adult rabbit consumes an average of 140 to 170 grams of food per day. The maximum percent body weight loss for animals on restricted diets should not exceed 20% relative to age- and sex-matched ad libitum fed controls.
- Due to variation in food requirements and nutritive status, use of average guidelines for food intake are not appropriate. Mature or obese animals can tolerate greater food restriction than their young or thin counterparts.
- If weight loss is expected this will need to be outlined in the IACUC approved protocol. In addition to appropriately determine percentage weight loss, weight should be compared to age matched controls fed ad libitum or based on age matched growth data from the vendor.
- Body condition score can also be used to assess health status (Appendix 1)
- Ideal score is 3/5; scores of 2/5 or less are considered under-conditioned to emaciated.
- Scores lower than 2/5 must be justified in IACUC protocol
- The amount of food and/or water consumed should be measured.
For Water Restriction Only
- The estimated daily water intake in an adult mouse is 15 ml /100g body weight and in an adult rat is 8-10ml/100g body weight. This amounts to 25% and 10% of the body weight of mice and rats, respectively. Rabbits consume 50-150 mls/kg of water daily, an amount that varies across breeds, age, sex, health and type of food available.
- These values may be used as a starting point to determine regulation or restriction parameters. However, individual variation in water consumption based on factors such as strain, sex, age, and health status must be taken into account when establishing fluid regulation or restriction paradigms.
- Mice, being one logarithmic unit smaller in body weight than rats, have correspondingly higher Basal Metabolic Rates (BMRs) and correspondingly faster water turnover. Mice are therefore less tolerant of water restriction and PIs must be aware of extending observations made on rats to mice.
- Skin turgor should be evaluated to assess the animal's hydration status when water is restricted.
- Solid and liquid waste should be evaluated to assess the animal's hydration status and physiologic compensation for fluid regulation if applicable.
- Rough hair coat and sunken eyes are indicators of dehydration.
Appendix 1
Table 1. Rodent Body Condition Score
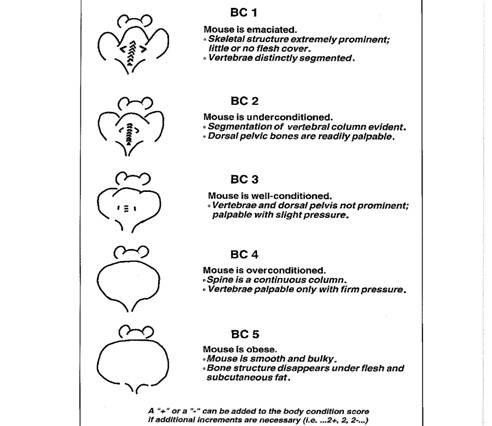
Ullman-Cullere MH, Foltz CJ. 1999. Body condition scoring: a rapid and accurate method for assessing health status in mice. Laboratory animal science 49:319-323
References
- NRC 2011. Guide for the care and use of laboratory animals, 8th edition. Washington: National Academies Press.
- NRC 2003. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington: National Academies Press.
- Bekkevold CM, Robertson KL, Reinhard MK, Battles AH, Rowland NE. 2013. Dehydration Parameters and Standards for Laboratory Mice. J Am Assoc Lab Anim Sci 52: 233-239.
Approved: December 2012
Revisions Approved: October 2017, 3/2018, July 2022, July 2023, April 2024