Veterinary Recommendations for Anesthesia and Analgesia
This Guidance Document is to establish recommendations for anesthesia and analgesia for use in research animals.
PRINCIPLES OF ANESTHESIA AND ANALGESIA
- The proper anesthetic and analgesic agents must be used in order to eliminate or reduce the potential for pain and distress during the perioperative period.
- Withholding anesthesia or analgesia must be justified and approved in the IACUC protocol.
- A multimodal approach to analgesia should be employed to offer the best broad-spectrum pain control possible. This includes the use of different categories of analgesics in combination to address different sources of pain perception/stimulation.
- For example, a surgical procedure may use a local anesthetic block of lidocaine and bupivacaine at the incision site, and systemic administration of an NSAID for inflammatory pain and an opioid.
- According to the 8th edition of the Guide for the Care and Use of Laboratory Animals (NRC), "Guidelines for the selection and proper use of analgesic and anesthetic drugs should be developed and periodically reviewed and updated as standards and techniques are refined."
ANESTHESICS
- Inhalant Anesthetics
- The inhalant anesthetics include gases such as isoflurane and sevoflurane. These anesthetics require an anesthetic machine set-up. In addition, use of a scavenger system is required to prevent personnel exposure to the waste anesthetics. For short, non-surgical procedures, it may be possible to administer inhalant anesthesia via a drop jar. For this procedure a cotton ball or gauze soaked with the anesthetic is placed in a jar with the animal. This procedure must be performed under a chemical fume hood, the animal CANNOT contact anesthetic, and can only be used for minor quick procedures (e.g., genotyping, injections, tumor implantation (trocar), drug pellet or microchip implantation). Animals will recover quickly after removal from jar.
- Advantages: safe and reliable, predictable and rapid control of anesthetic depth, not controlled substances
- Disadvantages: induction must be closely monitored, personnel training, special equipment required, potential risk to staff (if not appropriately scavenged)
- Injectable anesthetics
- Injectable general anesthetics include ketamine/xylazine and pentobarbital. Most of the commonly used agents are administered via intraperitoneal (IP) injection.
- Local anesthetics are often delivered subcutaneously along the incision site. They could also be used in nerve blocks or epidural administration. Local anesthetics are not adequate as the only analgesic for any surgical procedure unless scientifically justified in the protocol.
- Advantages: They can be used without expensive supporting equipment such as the anesthesia machines required with the use of inhalants, they are easily transported, and are relatively inexpensive.
- Disadvantages: Prolonged recovery times. The animal will have to metabolize the drug in order to completely recover from anesthesia. In addition, once the agent is injected, the anesthetic depth cannot be adjusted throughout the procedure except to achieve a deeper anesthetic plane by giving additional drug if the animal demonstrates signs of arousal. Animals which are sick or compromised may have a difficult time with these anesthetics due to changes in their ability to metabolize the drugs. Also, many of the commonly used injectable anesthetics are controlled substances which will require the laboratory comply with all rules regarding controlled substances and obtain a DEA license. DLAR does not provide controlled substances to laboratories for experimental procedures.
- Monitoring Anesthetic Depth
- Anesthetic depth should be gauged prior to conducting any surgical manipulation and throughout the surgical procedure. Loss of reflexes (e.g., pedal, corneal, palpebral) can be used to assess appropriate anesthetic plane. There should not be a response to toe pinch. Signs of inadequate anesthetic depth include purposeful movement, reflexes present, response to painful stimulus, or twitching whiskers. Equipment (e.g. pulse oximeter) may also be used to monitor depth of anesthesia. Changes in heart rate, respiratory rate, or blood pressure may indicate whether an animal is at too light or too deep of an anesthetic plane. Depth of anesthesia should be assessed every 10-15 minutes during surgery.
ANALGESIA
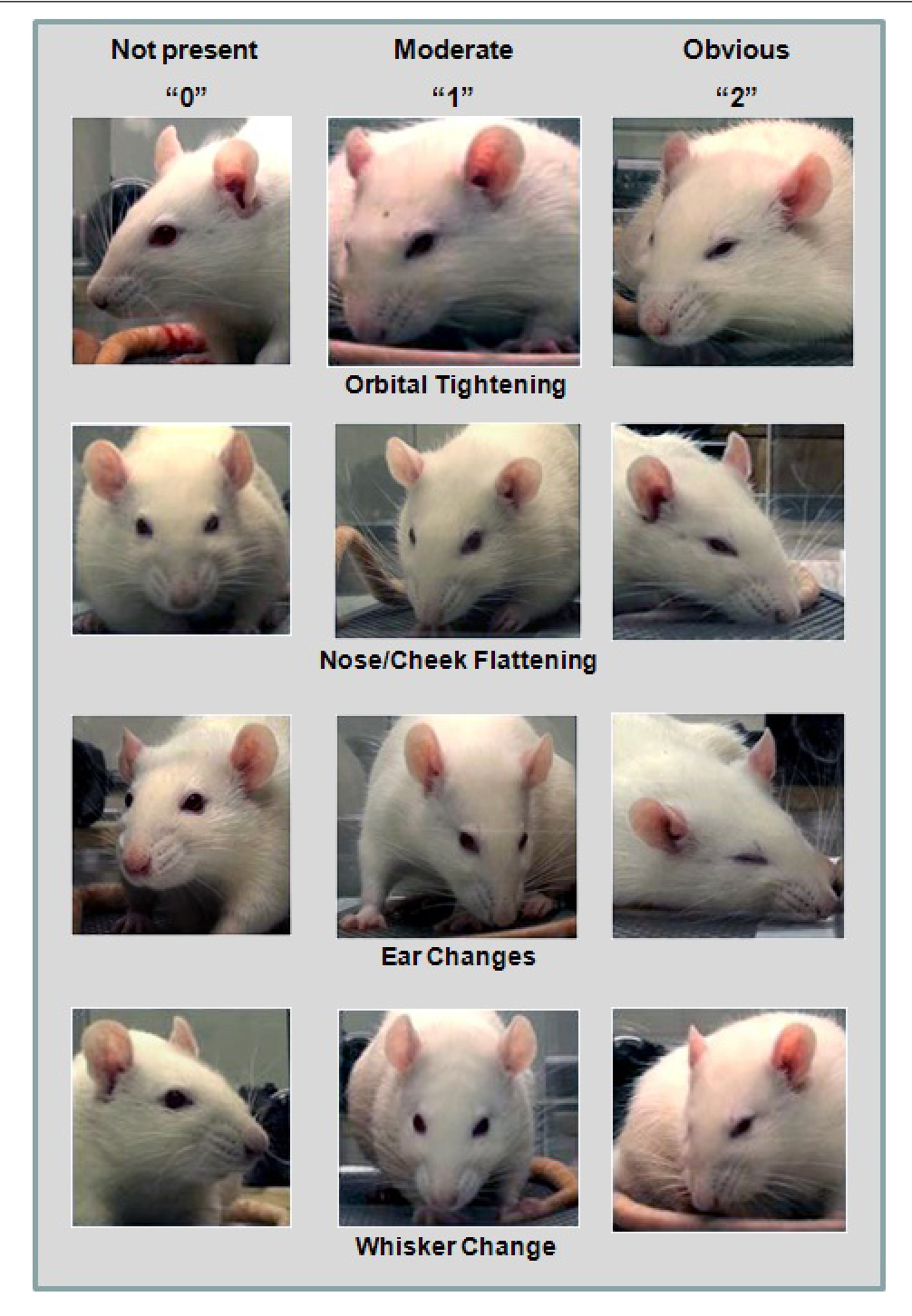
1. Pain assessment (Figures 1-2) and analgesia
Assessing pain is difficult in animals. Some animals are species of prey and are adapted to hide signs of pain and distress. Clinical signs associated with pain are species specific, but some common signs of all species include changes in appearance such as hunched, scruffy, porphyrin staining (rats/mice), or changes in activity, including less active or inactive, hyperactive or pacing, abnormal postures such as back arching, belly pressing, wound guarding, or writhing. Also, decreased appetite, isolation from cage mates, exaggerated or decreased response to handling, vocalization can be used to gauge pain and distress. Recently, there has been some focus on assessing rodent facial expression or grimace scale in order to assess pain. It is important to pay close attention to the animal's appearance and behavior post-surgery in order to observe subtle changes that may indicate the need for additional pain management.
Analgesia should be administered to ALL surgical animals unless otherwise justified in the protocol. It is recommended that the initial dose of analgesics is administered prior to the surgical procedure (i.e., pre-emptive analgesia). If there is concern regarding an animal's clinical condition post-surgery and additional analgesia is necessary, contact DLAR veterinary staff for further guidance on treating the animal.
Whenever possible multimodal analgesia is recommended. This involves providing a more 'balanced analgesia' through multiple methods or modalities. Local anesthetics at the incision site are often used in conjunction with a stronger opioid or NSAID analgesic.
RECOMMENDATIONS BY PROCEDURE
See specific doses, routes, and frequencies in the formularies below.
- Rodent laparotomy (example major surgery)
- Anesthetic induction/maintenance with appropriate anesthetic (isoflurane, ketamine/xylazine, pentobarbital)
- Administer opioid analgesic (e.g. buprenorphine, Ethiqa XR) prior to surgery. NSAIDs (e.g. Carprofen, Meloxicam) could be used as supplemental analgesia, but not recommended as sole analgesic agent for major surgical procedures.
- Consider using a local anesthetic along incision site for multimodal analgesia.
- Analgesics should be given for at least of 48 hours post-operatively.
- Rodents are monitored closely thereafter to evaluate for signs of pain, and additional analgesia is given until evidence of pain is no longer present.
- Rodent subcutaneous implant or vascular catheter placement (example minor surgery)
- Anesthetic induction/maintenance with appropriate anesthetic (isoflurane, ketamine/xylazine pentobarbital)
- Administer NSAID or opioid analgesic prior to surgery.
- Consider using a local anesthetic along incision site for multimodal analgesia.
- Analgesics should be given for at least 24 hours post-operatively.
- Rodents are monitored closely thereafter to evaluate for signs of pain, and additional analgesia is given until evidence of pain is no longer present.
- Neonatal Rodents (mice and rats): Neonatal rodents must have adequate anesthesia and analgesia when undergoing surgical procedures. It is important to balance safety with effectiveness when using anesthetics in neonatal animals. Neonates have an immature hepatic/renal system which can lead to prolonged anesthesia and a narrow margin of safety when using injectable medications. Inhalant anesthetics (isoflurane/sevoflurane) or hypothermia (<7 days of age) are the recommended methods of anesthesia in neonatal rodents. Opioid analgesics have been used successfully in neonatal rodents. However, these drugs should be administered at the lower end of the published dose range to avoid complications.
- Non-Rodent Species
- A protocol planning meeting with a veterinarian is required for all large animal surgical or invasive procedures which require anesthesia.
- The veterinarian will provide recommendations on an appropriate anesthetic/analgesic protocol.
Hypothermia Anesthesia
| Age | Altricial rodents up to 7 days old |
|---|---|
| Induction | 2-4 minutes. Protect pup by placing into finger of a glove or paper lined test tube. This will avoid skin damage caused by direct contact with ice/cold water. Immerse pup in ice water or place on crushed ice (2-3ËšC or 35-37ËšF). Observe pups closely during induction. |
| Maintenance | Up to 15 minutes anesthesia. Remove pups from ice bath when adequate anesthesia is achieved (immobile/lethargic). The pup can be maintained by placing on an ice pack covered with latex/paper towel. |
| Recovery | Up to 1 hour. Avoid rapid warming during the recovery period. Recommend re-warming using an incubator set at 90-95ËšF (32-25ËšC) or in a paper-lined cage set over a circulating warm water blanket. Electric heating blankets and heat lamps are not recommended. |
| Additional Considerations | Fiber optic lighting should be used during surgical procedure to help maintain hypothermia (incandescent lamps can warm surgical field). Steps should be taken to avoid rejection by dam post-surgery. Recommend: remove blood/disinfectants from pup after surgery (wipe with wet gauze and dry), make sure neonate is completely recovered (warm, pink, breathing, moving), place neonates in bedding/nesting material from home cage to obtain appropriate scent, and return neonates as a group to home cage. |
Mouse and Rat Formulary | ||||||
|---|---|---|---|---|---|---|
| Analgesics | Dose (mg/kg) | Route | Freq | Comments | ||
| Mouse | Rat | |||||
| Acetaminophen Oral dose Water bottle |
1-2 mg/ml drinking water | 100-300
6 mg/ml drinking water | PO | q4h | Not adequate as a sole analgesic except for very minor pain. May be combined with another class of analgesic for post-op pain.
Change water every other day. Water must be placed on cage 48 hours prior to painful procedure in order for rats to acclimate. | |
| Buprenorphine | 0.05 - 0.1 | 0.01 - 0.05 | SC | q12h | Excellent for moderate to severe pain (major surgical procedures). | |
| Buprenorphine ER | 1-1.2mg/kg | 1-1.2mg/kg | SC | g72h | Excellent for moderate to severe pain (major surgical procedures). One does lasts up to 72 hours. | |
| Ethiqa XR | 3.25 | 0.65 | SC | q72h | Excellent for moderate to severe pain (major surgical procedures). One dose lasts up to 72 hours. | |
| Carprofen | 5 | 5 | SC | q24h | Good for mild to moderate pain (minor surgical procedures). | |
| Carprofen or Meloxicam tabs (Bioserv®) | PO | q24h | Administration based on manufacturer recommendations. Mouse and rat formulations of Meloxicam available. Single rodent formulation available for Carprofen. | |||
| Meloxicam | 1 - 2 | 1 - 2 | PO, SC | q24h | Good for mild to moderate pain (minor surgical procedures). | |
| Meloxicam SR | 2 | 4 | SC | m: q24h; r: q72h | Provides stable course of analgesia after a single administration. Compounded product from zoopharm. Since mice metabolize meloxicam at a rate 20x higher than rats, therapeutic levels of sustained release may not exceed 24 hrs | |
| Tramadol | 20 - 40 | 5 - 20 | IP | For chronic or severe pain. Literature does not define recommended dosing interval in rodents. In other species administered up to TID. Start at BID dosing. | ||
| Anesthetics | Dose (mg/kg) | Route | Duration | Comments | ||
| Mouse | Rat | |||||
| Recommended: Isoflurane | 2 - 5 % | 2 - 5 % | Inhalation | Gold standard anesthetic. Must have appropriate equipment to use safely (precision vaporizer and scavenging). | ||
| Ketamine/ xylazine | 90 - 120 10 | 40-80 5-10 | IP | 30 - 45 min | Ketamine combinations are the next best anesthetic if isoflurane cannot be used. Do not re-dose xylazine; if additional dose needed provide ~1/3 dose ketamine | |
| Ketamine/ medetomidine | 50 - 75 1 - 10 | 60 0.4 | IP | |||
| Ketamine/ xylazine/ acepromazine | 100 2.5 2.5 | 40 8 4 | IM, IP | |||
| Pentobarbital | 30 - 90 | 30 - 60 | IP | 60 - 120 min | Not readily available. High cost associated with pharmaceutical grade formulations. | |
| Tribromoethanol | 250 | Not recommended | IP | Very short term anesthesia, not recommended for survival procedures; inappropriate storage or mixing may result in toxicity. Must scientifically justify use in protocol. | ||
| Reversal Agents | Dose (mg/kg) | Route | Duration | Comments | ||
| Mouse | Rat | |||||
| Yohimbine | 0.2 | 0.2 | IP | NA | Reverses xylazine | |
| Atipamezole | 1 | 1 | SC | NA | Reverses xylazine and medetomidine | |
| Local Anesthetics | Dose (mg/kg) | Route | Duration | Comments | ||
| Mouse | Rat | |||||
| Bupivacaine/ Lidocaine mixture | 1.5 mg/kg 0.5 mg/kg | 1.5 mg/kg 0.5 mg/kg | SC | 4 - 8 hrs | Slow onset, long duration | Mix together in same syringe for infiltration around incision |
| <1 hr | Rapid onset, short duration | |||||
Figure 1. Mouse Grimace Scale2
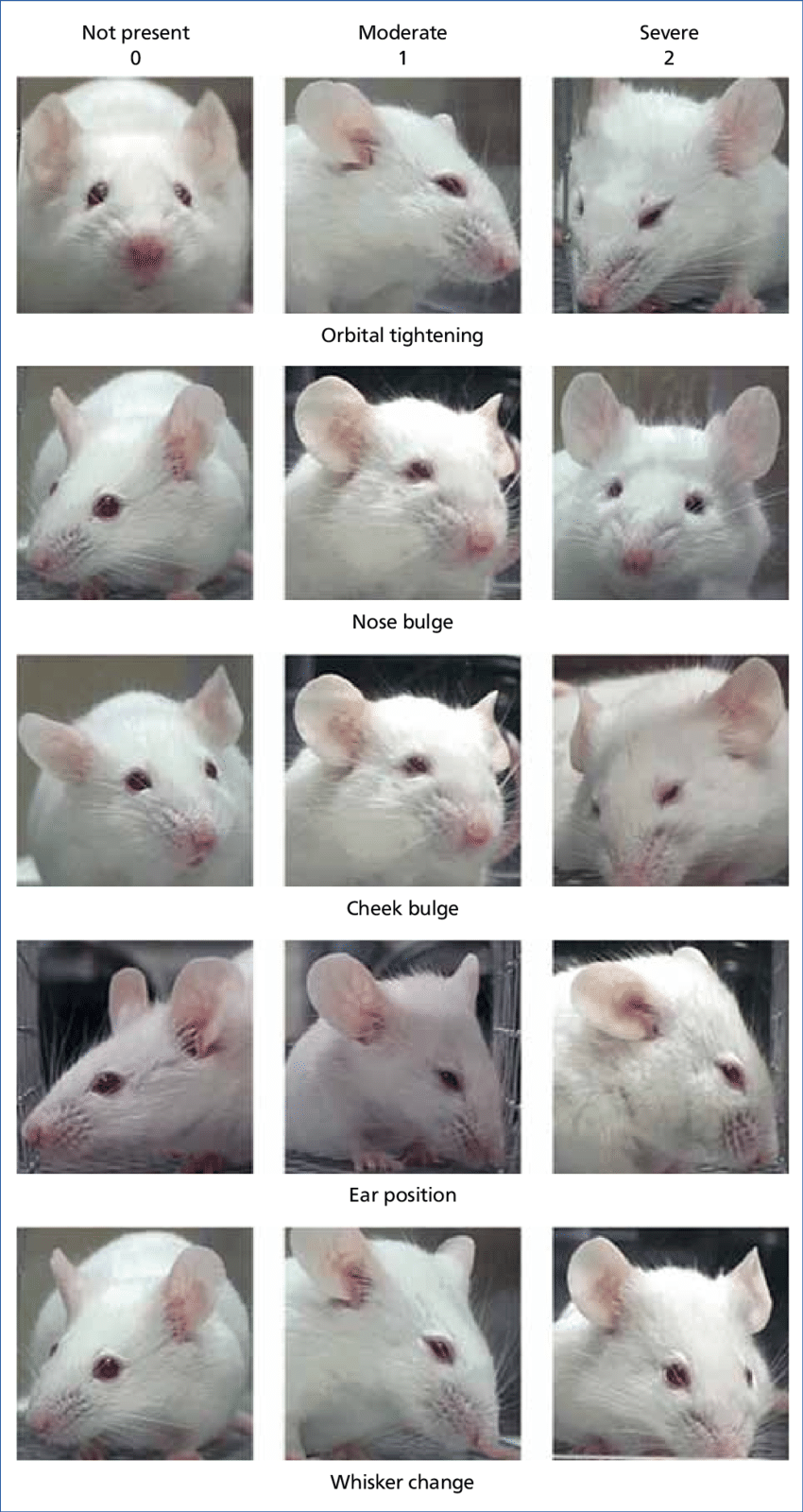
Figure 2. Rat Grimace Scale3
References
Primary reference:
Fish RE et al. Anesthesia and Analgesia in Laboratory Animals, 2nd edition, 2008.
Other references:
1. Carpenter J. Exotic Animal Formulary, 4th ed, 2012.
2. Langford, D., Bailey, A., Chanda, M. et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7, 447449 (2010) https://www.nature.com/articles/nmeth.1455
3. Sotocinal SG, Sorge RE, Zaloum A, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. Published 2011 Jul 29. https://api.semanticscholar.org/CorpusID:9182406
4. Kuehn N. North American Companion Animal Formulary, 9th ed, 2010.
Date: December 2013
Revised: October 2019, May 2020, February 2022, January 2024