Induction and Monitoring of Experimental Autoimmune Encephalitis (EAE) in Rodents
BACKGROUND
This document is designed to provide information for investigators and animal care staff regarding the use of rodent models of experimental autoimmune encephalitis (EAE) at Wayne State University. These models have variable presentations that are dependent upon a number of factors including induction method and animal strain but they all produce some degree of central nervous system impact that most often presents as progressive, ascending paralysis. Because of the variability in onset and presentation and potential welfare implications, close monitoring and provision of supportive care is necessary for EAE animals.
IACUC Procedure
Scoring Systems
Clinical signs associated with progression of ascending paralysis in EAE are commonly graded using a six-stage scale of 0-5, with "0" indicating a clinically normal animal and "5" indicating paralysis of all four limbs (quadriplegia). Other functional scoring systems may be utilized if they are clearly defined in the protocol and posted clearly in the animal room for reference by DLAR staff.
Body condition score (BCS) must be used to evaluate the general physical condition of EAE animals and used to compliment the functional score. The standard rodent BCS assigns animals a score ranging from 1-5 based upon body fat and muscle mass over the caudal dorsum (see Figure 1). A score of "1" indicates emaciation and a score of "5" indicates a grossly obese animal. Ideal body condition is represented by a score of "3".
Animal Use Category
All animals used in EAE experiments must be placed into Category E in the IACUC protocol. This assignment is required as animals are expected to experience varying degrees of paresis/paralysis and may develop neuropathic pain, similar to human multiple sclerosis patients, during disease progression.
Record-keeping
A log must be maintained by laboratory personnel for all animals beginning at the time of inoculation. The dated log must include all relevant monitoring information including animal weight, BCS, EAE score, hydration and bladder status, treatments administered, and personnel initials. Required parameters and assessment frequency are described in the Animal Care section of this document. The log or a current copy must be kept in the vivarium, preferably in the animal housing room, and accessible to DLAR staff at all times. The IACUC developed an "EAE Mouse Monitoring Form" that can be utilized.
Animal Care
Specific assessments, frequency, and prescribed treatments will be triggered at the start of experiments and depend upon the EAE score as disease progresses. The care described here is the standard minimum requirement and any deviations must be included in the approved IACUC protocol.
- At the time of inoculation:
- Initial weight must be recorded in the logbook for each animal.
- The name of the substance(s) used to induce the EAE model and the date of inoculation must be recorded on the cage card. This must be done whenever additional inoculations are done, if applicable.
- If chemical hazards, as defined in the protocol, are used for inoculation containment housing procedures must be observed. See the building technician leader for additional information.
- EAE Score = 1 2 (Flaccid Tail, Hind Limb Weakness):
- When clinical signs are expected to begin, laboratory staff must begin monitoring at least once daily, including weekends and holidays.
- Daily monitoring must include overall activity, EAE score, and hydration status.
- Animals with increased skin turgor (skin tenting) must receive 1 cc of 0.9% saline subcutaneously once daily.
- Weight and body condition score must be recorded at least twice weekly.
- Provisions for increased food and water access must be made:
- Long sipper tube placement on water bottles
- Pelleted food placed on floor of cage
- Moist chow or gel diets are recommended and must be placed if and animals in the cage have a BCS 2 or have lost > 10% of their initial body weight.
- EAE Score = 3 (Hind Limb Paralysis, Urinary Incontinence)
- All monitoring described above must continue.
- Animals must be monitored daily for dermatitis, urine scald, penile prolapse (males) or tail lesions. The appearance of any of these conditions must be reported to DLAR staff.
- The bladder must be palpated at least three times a week for bladder atony (inability to urinate).
- Signs of atony include a large bladder, urine staining of the fur, and/or distended abdomen.
- Once bladder atony is identified, affected animals require twice daily, manual expression to evacuate urine (8 to 12-hour interval). DLAR technicians and trainers can provide guidance on the identification of bladder atony and proper expression technique.
- Place animals on Pure-O'cel bedding.
- Moist chow or gel diets must be placed on floor of cage.
- EAE Score = 4 (Forelimb Weakness with Hind Limb Paralysis)
- All monitoring described above must continue.
- Weight and BCS must be recorded daily.
- Animals that maintain a score of 4 for more than 24 hours must be euthanized unless described and justified in the IACUC protocol. These animals may require additional fluid, nutritional and heat support as determined by DLAR staff.
- EAE Score = 5 (Paralyzed Front and Hind Limbs)
- Animals exhibiting these signs must be euthanized no later than the end of the day.
- Animals with additional evidence of morbidity (recumbent, poorly responsive or failure to right, abnormal breathing) must be euthanized immediately.
Humane Endpoints
Specific endpoints for EAE are study and presentation specific must be described and justified in the IACUC protocol.
In general, animals that lose > 20% of their baseline weight or have a BCS of 1 must be euthanized by the end of the day. Certain EAE models have a well-documented, chronic, relapsing/remitting course characterized by the development of disease with intervals of recovery. In models with this presentation it may be appropriate to permit a higher degree of weight loss with the expectation that animals will recover both function and body condition in time. This exception to the 20% endpoint, and any additional supportive measures required during the period of high weight loss, must be discussed with a DLAR veterinarian and must be described and justified in the IACUC approved protocol. This exception does not apply to models with a progressive course that lack a period of remission.
Figure 1. Body Condition Scoring System for Rodents
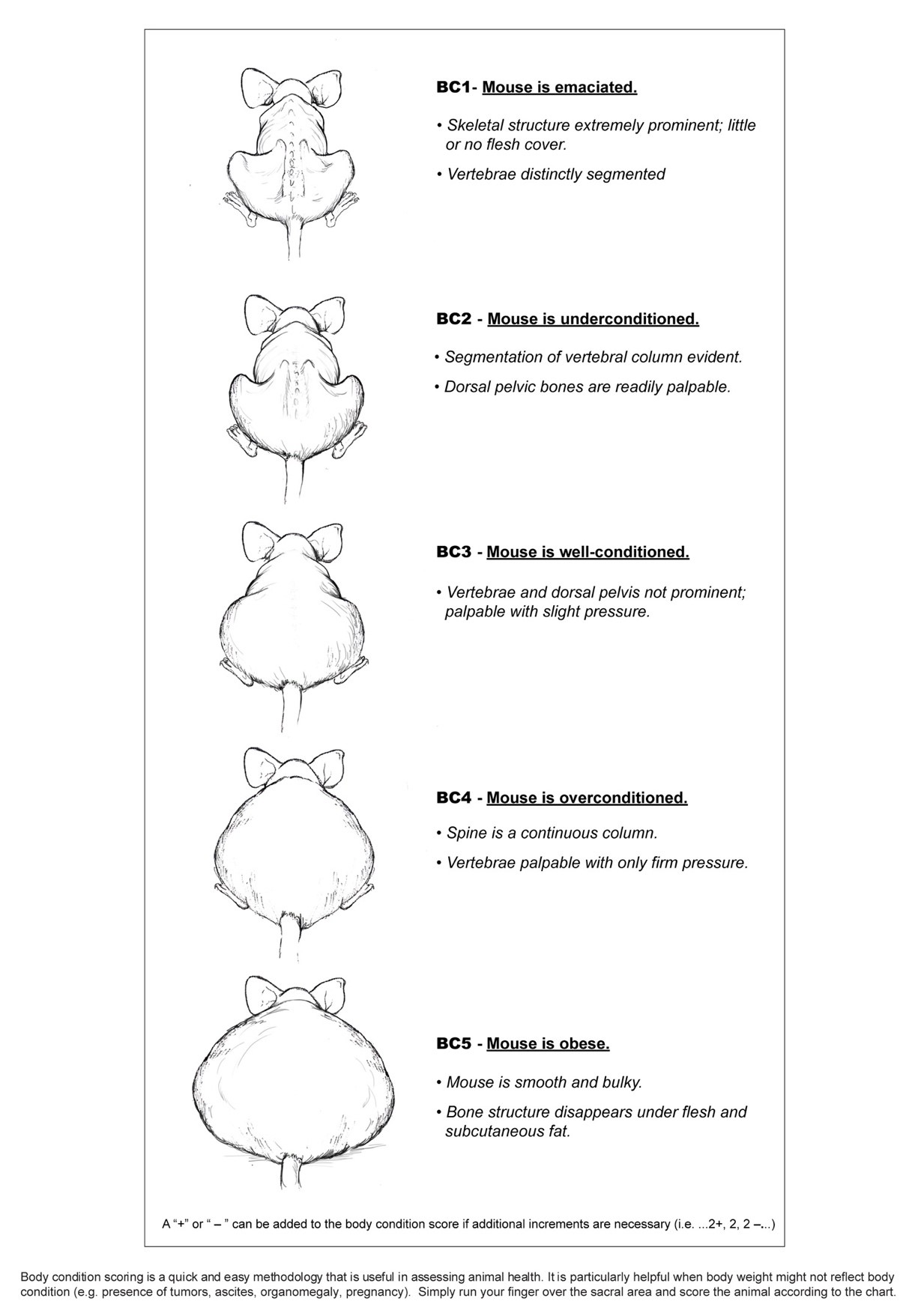
From: Burkholder et al, 2012 Health Evaluation of Experimental Laboratory Mice, Cur Protocol Mouse Biol, Vol 2 pp. 145-165. DOI:10.1002/9780470942390.mo110217.
Approved: April 2020